| 1 | a) | K+Cl– | b) | Mg2+(Br–)2 | c) | Ca2+S2- |
| d) | Ca2+(H–)2 | e) | (Na+)2O2- | f) | Li+Cl– | |
| g) | Pb4+(O2-)2 | h) | Al3+(Br–)3 | i) | Cs+Cl– |
| 2. | |
| a) | SiC |
| ii) Covalent network compounds – breaking strong covalent bonds requires a lot of energy | |
| b) | i) does not have freely moving charged particles/ |
| 3. | |
 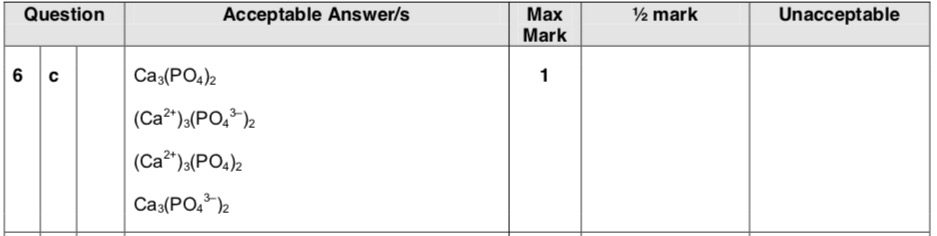 |
|
| 4. | |
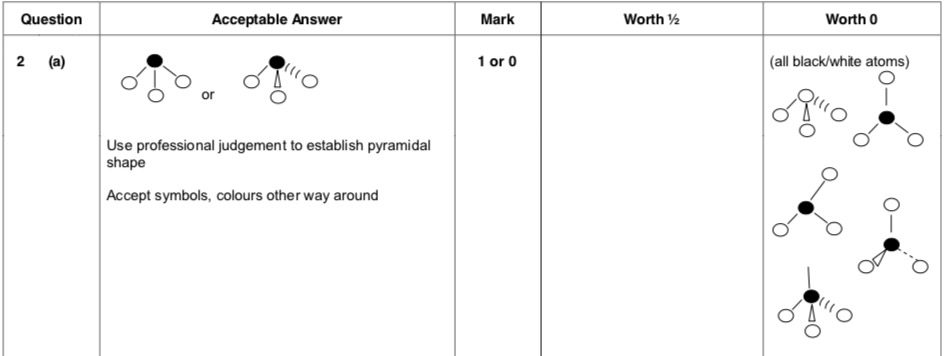 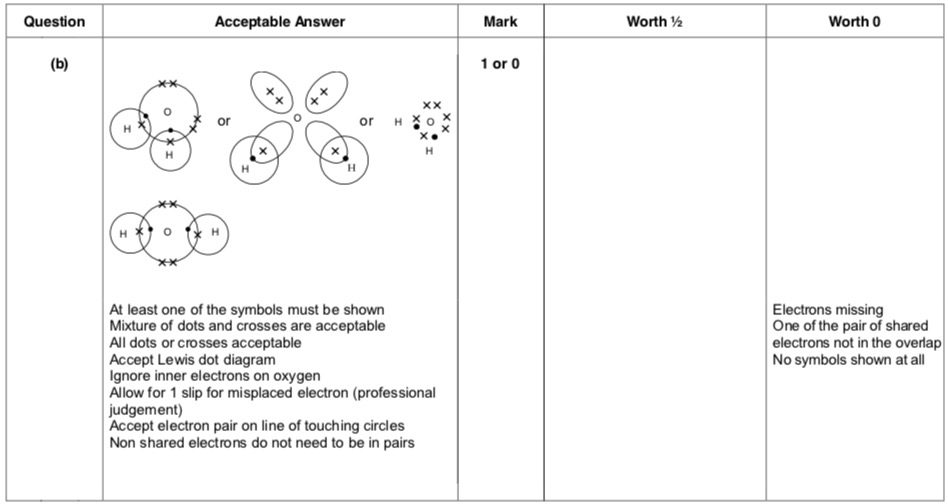 |
|
| 5. | |
| i) | C3H9N |
| ii) | molecule |
