| 1. | C |
| 2. | B |
| 3. | D |
| 4. | B |
| 5. | C |
| 6. | C |
| 7. | B |
| 8. | B |
| 9. | C |
| 10. | C |
| 11. | C |
| 12. | |
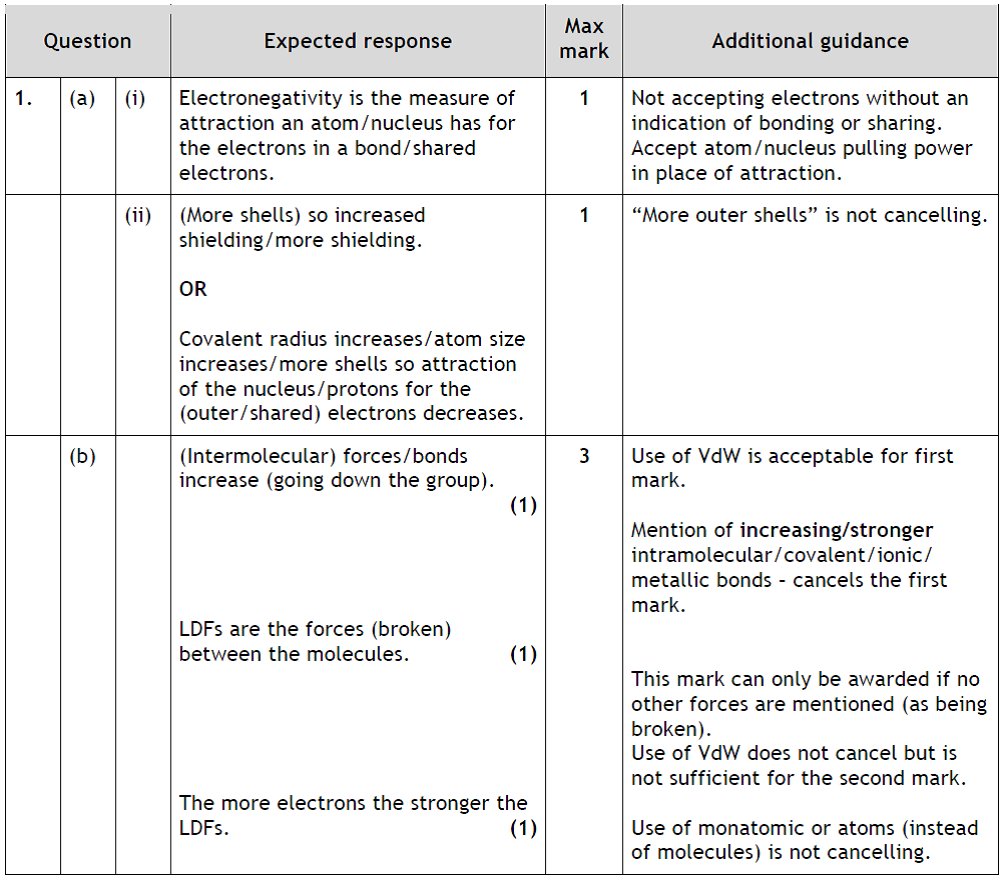 |
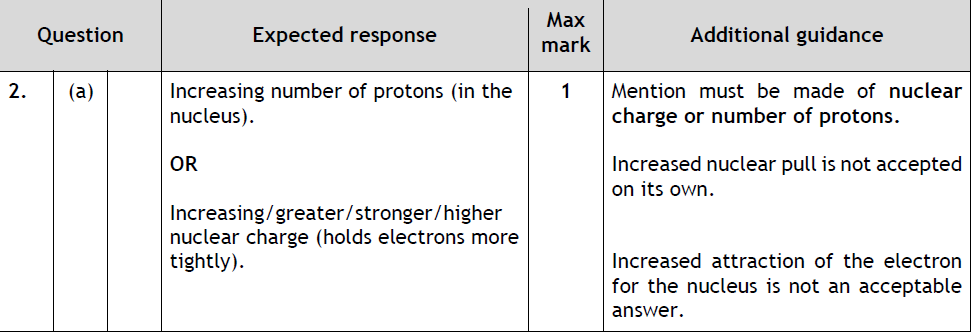
|
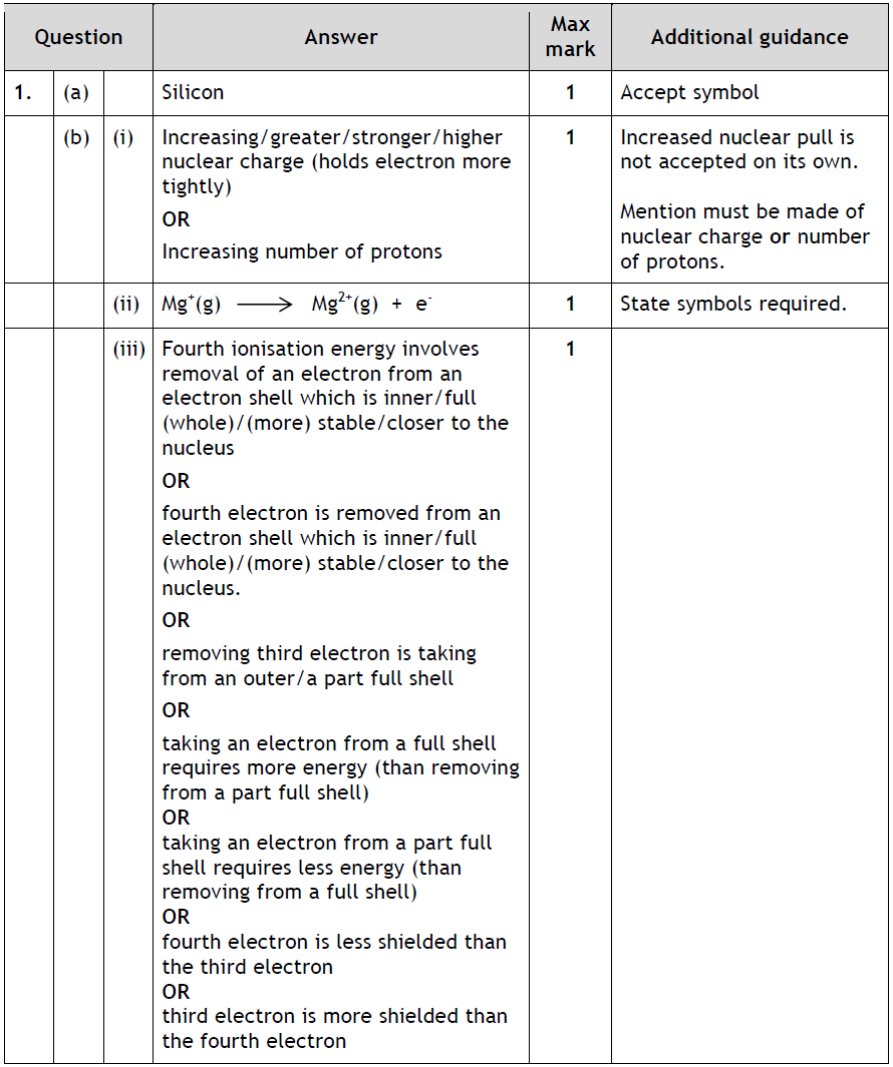
| 13. | |
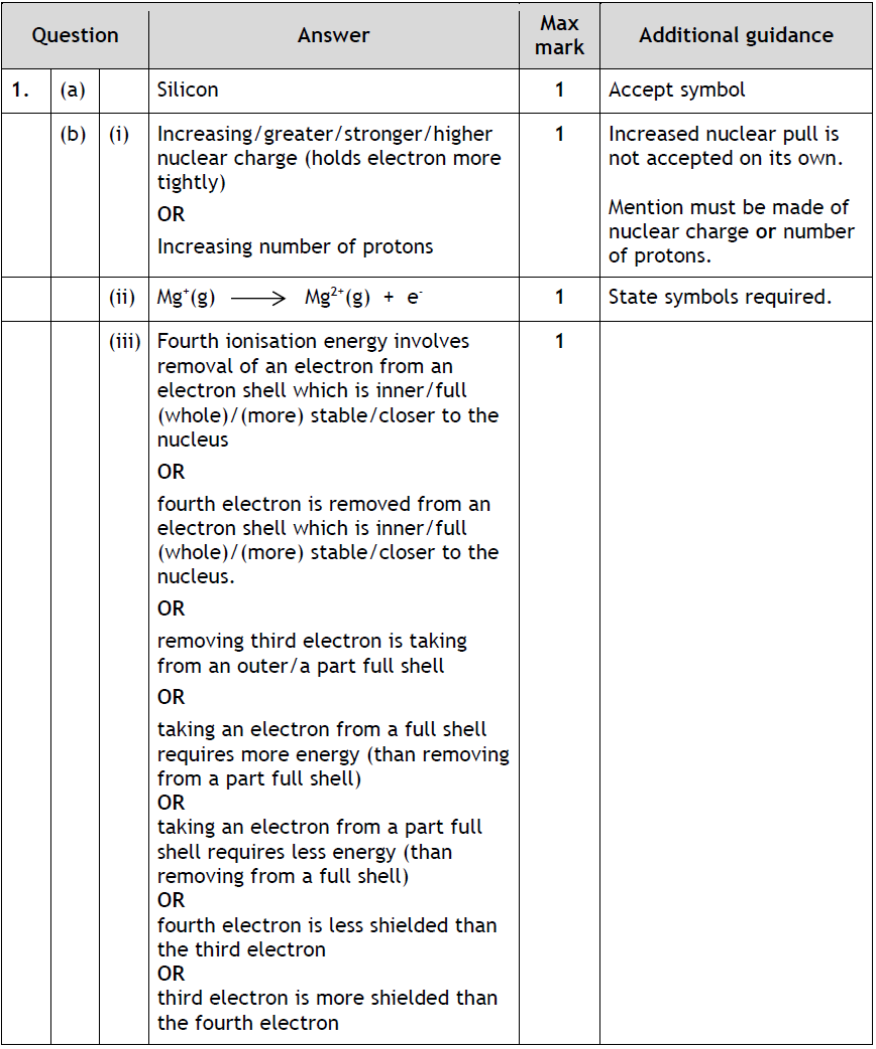
 |
|
| 14. | |
 |
|
| 15. | |
 |
|
| 16. | |
  |
|
| 17. | |
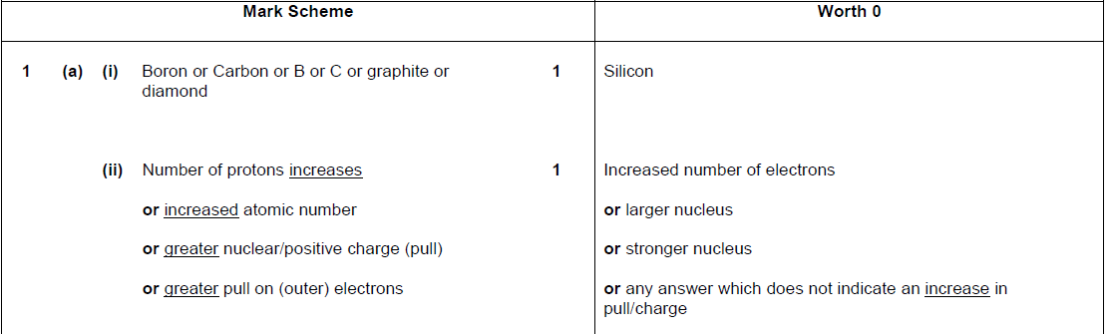 |
|
| 18. | |
| A sodium ion is formed by loss of a single, outer electron, leading to a reduction in the number of occupied energy levels / shells. 2,8,1 becomes 2,8.
A chlorine atom is formed by the addition of a further electron, filling the outer energy level/ shell. 2,8,7 becomes 2,8,8. The chlorine ion has 3 occupied energy levels/ shells, the sodium has only two. |
|
| 19. | |
| As the size of the ion increases, the enthalpy of lattice formation decreases | |
| 20. | |
 |

